4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
December 21, 2023 at 8:30 AM
Dr. Andrea Gruszecki, ND
:
December 21, 2023 at 8:30 AM

The season of stress is upon us. The winter months are host to a dizzying number of holidays, secular and not, for a variety of cultures around the world. With rare exception, holiday celebrations involve dietary excess and visits with friends and family. While a happy holiday is a “positive stressor”, happy or not, most holidays involve the added stress of shopping, house-cleaning, visitors, and preparing/partaking of holiday foods. All of the added holiday stress, positive or not, can have a negative impact on digestion and microbiome diversity. Holidays may also involve dietary stressors, as the urge to “cheat” and indulge in holiday treats that can increase the risk of exposure to food allergens or sensitivities, and toxic exposures such as chemical food/beverage additives or mycotoxins. These holiday stressors and environmental exposures can increase levels of systemic and neuroinflammation. Systemic inflammation is associated with cognitive decline and neuroinflammation is associated with Alzheimer’s disease, Parkinson’s, and other neurological disorders. Both types of inflammation are associated with changes in the gastrointestinal microbiome and disrupted intestinal permeability.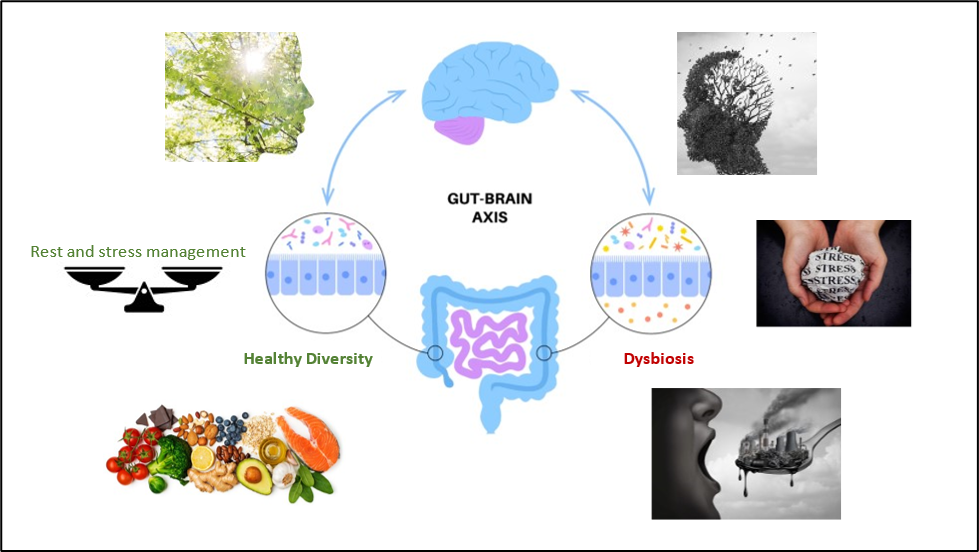
Psychological stress has been shown in multiple human and animal studies to alter microbiome diversity, mitochondrial, and gut barrier functions. Significant changes in the bacterial phyla, families and genera can be found in stressed gut microbiomes in bacteria important for health, barrier function, and appropriate immune system responses. The studies all agree that, in general, stress induces an overall decrease in beneficial bacteria and an increase in dysbiotic bacteria. The gut bacteria that affect the peripheral and central nervous system act either through their effects on the immune system or via the production of neuro-active metabolites:
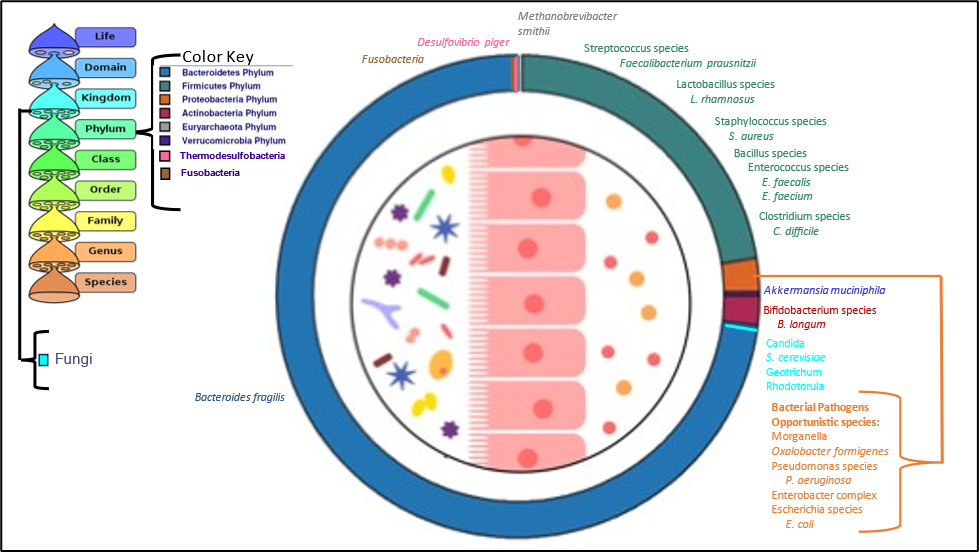
The loss of beneficial bacteria and their immune-supportive effects, the inhibition of normal digestive functions, and the resultant increase in gut permeability contribute to the passage of larger foreign inflammatory dietary proteins and increased amounts of bacterial lipopolysaccharide (LPS) into the bloodstream. Increased alcohol use, combined with increased dietary fats and carbohydrates can further exacerbate gut barrier dysfunction, mitochondrial dysfunction, and the growth of dysbiotic microbiome species. Bacteria that decrease with stress may include the Verrucomicrobia phylum (Akkermansia species), and several members of the Firmicutes phylum, while bacteria that increase with stress may include methanogens (Methanobrevibacter smithii). While not specifically demonstrated yet in human studies, most animal studies demonstrate an expansion of Proteobacteria during stress. Bacteria from the Proteobacteria phylum include pathogens and dysbiotic species associated with local and systemic inflammation.
A loss of beneficial bacteria or an increase in dysbiotic species can also contribute to increased intestinal permeability. The same factors that disrupt gut mucosal permeability (inflammation and stress) can also disrupt the blood-brain barrier and allow foreign materials into the central nervous system. In addition to inflammatory proteins, animal studies indicate that once LPS crosses the blood-brain barrier, it is difficult to remove and tends to remain in the brain causing local inflammation studies associate with increased fatigue, cognitive changes, and neurological disorders. This means that a while transient stressor may cause gut and brain barriers to “leak” for a few hours, the proteins and LPS that get trapped inside the central nervous system may contribute to long-term neuroinflammation and risk of cognitive decline or neurological disease.
Holiday treats are often higher in simple carbohydrates (sugar), fat, and salt and lower in fiber than everyday foods. Increased animal protein may also be part of holiday celebrations. Excess animal protein, sugars, salt, and fat are the components found in the pro-inflammatory “Western diet”. In addition to these macronutrients, commercially prepared holiday foods may contain food colorings, flavorings and other additives that can have an inflammatory effect on the gut mucosal lining and intestinal permeability and disrupt the bacteria of the gut microbiome. Holiday foods rich in grains, dried fruits and nuts may harbor high levels of mycotoxins and other mold proteins that can cause IgE allergic reactions and inflammation. Such shelf-stable foods may contain mycotoxins that disrupt gastrointestinal and mitochondrial function as well as the gut microbiome.
Stress, alcohol, and poor diet can also alter or inhibit normal mitochondrial functions essential for neurological health, appropriate immune responses, and cellular energy production. Mitochondrial dysfunction is also associated with cognitive changes and neurological disorders, as well as gut microbiome status and barrier functions. Long-term, increased stress, loss of microbiome diversity, and mitochondrial dysfunction may increase the risk of environmental or food allergy, which are also significantly associated with dementia risk.
Metabolites produced by gut bacteria, including LPS and short-chain fatty acids such as butyrate, can signal either inflammation or tolerance, tight gut barriers or leaky guts, etc. Gut cells in the colon use bacterial butyrate or acetate as fuel via mitochondrial -oxidation. Gut mitochondrial respiration, essential for cell energy production, can be inhibited by high levels of hydrogen sulfide gas. Recent studies have associated excess hydrogen sulfide gas and altered sulfur metabolism mediated by Akkermansia muciniphila and various sulfur-metabolizing species from the phylum Thermodesulfobacteriota with Parkinson’s disease. A. muciniphila was identified as an hydrogen sulfide gas producer in Parkinson’s patients, while the Thermodesulfobacteriota species was implicated in the production of sulfite, a known neurotoxin associated with Parkinson’s and an oxidative stressor.
‘Tis the season for holiday stress and overindulgence… or is it? A pro-active approach to the holiday can minimize the risk of neuroinflammation and help ensure a happy, healthy holiday season for all.
References:
Bostick JW, Schonhoff AM, Mazmanian SK. Gut microbiome-mediated regulation of neuroinflammation. Curr Opin Immunol. 2022 Jun;76:102177.
Bairamian D, Sha S, Rolhion N, Sokol H, Dorothée G, Lemere CA, Krantic S. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer's disease. Mol Neurodegener. 2022 Mar 5;17(1):19.
Borbolis F, Mytilinaiou E, Palikaras K. The Crosstalk between Microbiome and Mitochondrial Homeostasis in Neurodegeneration. Cells. 2023 Jan 28;12(3):429.
Daschner A. An Evolutionary-Based Framework for Analyzing Mold and Dampness-Associated Symptoms in DMHS. Front Immunol. 2017 Jan 9;7:672.
Hertel J, Harms AC, Heinken A, Baldini F, Thinnes CC, et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson's Disease. Cell Rep. 2019 Nov 12;29(7):1767-1777.e8.
Joh HK, Kwon H, Son KY, Yun JM, Cho SH, Han K, Park JH, Cho B. Allergic Diseases and Risk of Incident Dementia and Alzheimer's Disease. Ann Neurol. 2023 Feb;93(2):384-397.
Kim JH. Three principles for radiation safety: time, distance, and shielding. Korean J Pain. 2018 Jul;31(3):145-146.
Liew WP, Mohd-Redzwan S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front Cell Infect Microbiol. 2018 Feb 26;8:60.
Ma L, Yan Y, Webb RJ, Li Y, Mehrabani S, Xin B, Sun X, Wang Y, Mazidi M. Psychological Stress and Gut Microbiota Composition: A Systematic Review of Human Studies. Neuropsychobiology. 2023;82(5):247-262.
Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607-28.
Solanki R, Karande A, Ranganathan P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front Neurol. 2023 May 15;14:1149618.
Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH Jr, Selvin E, Windham BG. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019 Mar 12;92(11):e1256-e1267.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020 Jun;30(6):492-506.
Zhou X, Qiao K, Wu H, Zhang Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules. 2023 Jan 7;28(2):631.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...