4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
February 21, 2024 at 8:30 AM
Dr. Andrea Gruszecki, ND
:
February 21, 2024 at 8:30 AM

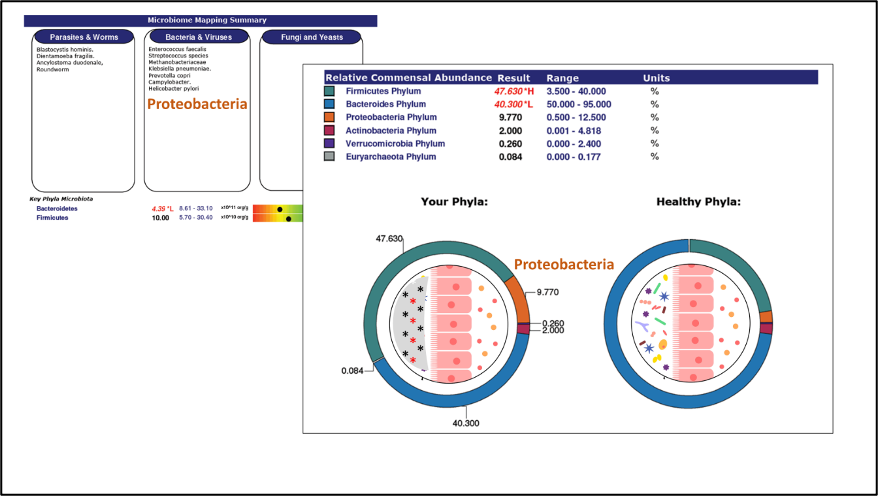
More than 30% of deaths worldwide involve cardiovascular disease (CVD). CVDs include coronary artery disease, stroke, heart failure and hypertension. Although established treatments and preventative care can reduce risks, experts still expect the mortality rates and financial burdens of CVD to increase. Recent studies indicate that the health of the heart and the health of the gastrointestinal system and its associated microbiome are closely associated: a disrupted GI microbiome may increase CVD risk factors such as inflammation. Human studies indicate that vascular inflammation and “hardening” of the arteries (atherosclerosis) is associated with an altered Firmicutes:Bacteriodetes ratio (increased Firmicutes phylum), Actinobacteria species from the gut microbiome can be found in atherosclerotic plaques in the body’s arteries, and that Helicobacter species and Proteobacteria microbiome abundance is increased in atherosclerotic patients.
Chronic inflammation is a known CVD risk factor and higher circulating levels of inflammatory cytokines (such as CRP and IL-6) are strongly associated with increased CVD and heart failure risks. An increasing number of studies indicate that, in addition to other pro-inflammatory risk factors such as diet, lifestyle and comorbid disease, the status of an individual’s gastrointestinal microbiome may represent an additional inflammatory risk factor. According to recent studies, two significant sources of increased inflammatory CVD risk are super-antigens and bacterial metabolites derived from dietary proteins. Active research in these areas includes in vitro (test tube), animal, and small human studies. It seems that the way to our hearts is through our gut and its microbiome. If this is so, then an evaluation of patient gastrointestinal function and gut microbiome diversity should be considered as necessary as blood pressure and hemoglobin A1c monitoring for those at cardiovascular risk.
The gastrointestinal microbiome is primarily composed of hundreds of different types of bacteria; smaller numbers of other microbes can be found in the gut microbiome including yeasts and bacteria-eating viruses (phages). Many of the compounds produced by the gut microbiome are essential for gut health and tolerant anti-inflammatory immune responses. These microbial compounds are signal molecules that “cross-talk” with other microbes or with the gut lining and underlying immune system.
The gut microbiome interacts with the gut-associated lymphoid tissues (GALT) found beneath the gut mucosal layer to program local and systemic immune responses via the cross-talk signals. Most immune cell priming occurs in the gut, and depending upon levels of gastrointestinal tolerance or inflammation, cytokine signals program naïve immune cells as tolerant T-regulatory (Treg), or inflammatory T cells (Th1, Th2, Th17).
The gut microbiome can be disrupted by the loss of beneficial bacteria or due to the overgrowth of dysbiotic species or pathogens. These disruptions can occur for a variety of reasons such as stress, diet, disease, toxic exposure, or environment. Once the gut microbiome is disrupted, inflammatory signaling increases. Without proper cross-talk from the gut microbiome inflammation, mucosal irritation and loss of intestinal permeability can develop. If intestinal permeability is compromised, then inflammatory compounds from the gut lumen can “leak” into the circulation and cause problems elsewhere in the body. Leaking super-antigens and bacterial protein metabolites from the gastrointestinal tract are now being associated with increased CVD risk.
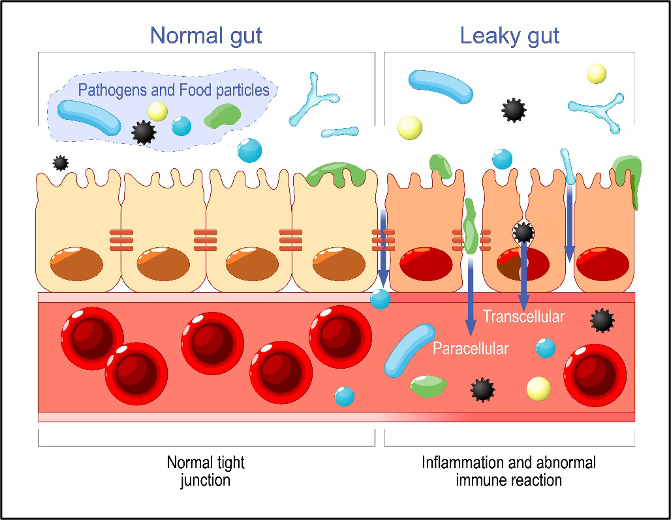
“Superantigens” are proteins produced by bacteria and viruses that are 20x more potent than ordinary antigens. Superantigens can induce B-cell proliferation, antibody production, and inflammatory responses and they can be produced both by pathogens such as Staphylococcus aureus and by some gut microbiome bacteria. In the gastrointestinal tract bacterial superantigens induce IgA antibody production, increasing the levels of secretory IgA in the gut lumen. Some superantigen-producing commensal microbiome bacteria have been associated with atopic disorders (eczema, asthma, allergy), lupus (SLE) nephritis, spondyloarthritis, and inflammatory bowel disease (IBD) in human studies. Once in circulation superantigens can bind directly to and activate cardiac mast cells, increasing local inflammation in the heart tissues and increasing CVD risk. In vitro (test tube) studies on heart muscle have demonstrated that super-antigen activation of cardiac mast cells induces the release of histamine and inflammatory cytokines. The study looked specifically at IgE activation, and higher levels of circulating IgE have been associated with increased CVD risk in multiple human studies.
As shown below, increased antibody production may result not only from gastrointestinal superantigens or allergy (IgE) but also from environmental sensitivity (IgA/IgG) exposures. IgA/IgG exposures at mucosal surfaces (gut, lung, skin) also induce antibody production. An excess of these circulating immunoglobulin (Ig)-antigen complexes activate and disrupt the function of the complement pathway.
Chronic complement activation increases systemic inflammation, another known cardiac risk factor. Complement activation on platelets specifically increases clotting and vascular inflammation. It is important to remember that mast cells also contain receptors for IgA and IgG antibodies as well as IgE receptors, and these other avenues of mast cell signaling may also prove important for heart health in the future. Decreasing exposure to allergens and sensitivities is an effective strategy for decreasing local and systemic inflammation. US BioTek offers both IgE allergy and IgA/IgG sensitivity testing for foods and inhalants to assist clinicians in allergy and sensitivity assessments.
Bacterial protein metabolites from the gastrointestinal tract include compounds such as trimethylamine N-oxide (TMAO) and phenylacetylglutamine (PAGln). Higher levels of circulating TMAO have been associated with changes in blood clotting, bile acid metabolism, vascular injury/inflammation, and macrophage activation. TMAO is a metabolite of both the liver and the gut microbiome; it is derived from dietary choline (animal products, cruciferous vegetables, nuts, seeds), carnitine (animal products), betaine, dimethylglycine (grains, meats), and ergothioneine (mushrooms, beans). Several gut bacteria have been associated with both atherosclerosis incidence and TMAO production including some species of Bifidobacterium, Clostridium, Fecalbacterium, Streptococcus, Klebsiella, and Escherichia coli.
Phenylacetylglutamine (PAGln) is another gut microbiome metabolite associated with heart failure, atherosclerosis, stroke, and heart attack in human studies. The PAGln metabolite is the result of phenylalanine metabolism by gut bacteria and is considered to be an independent predictor of CVD risk. A disrupted Firmicutes:Bacteroidetes ratio (increased Firmicutes phylum) and increased gut microbiome abundance of Citrobacter, Enterobacter cloacae complex, Escherichia, and Salmonella species were associated with significant loss of everyday function and increased symptoms of shortness of breath and angina in heart failure patients.
Another human study found increased circulating PAGln and altered microbiome diversity in patients with coronary artery disease (CAD). Stents are small tubes placed into blocked or narrowed blood vessels to normalize circulation. Compared to controls and CAD patients with functional stents, CAD patients with dysfunctional stent hyperplasia or stenosis (narrowing) had a decreased Firmicutes:Bacteroidetes ratio and increased abundance of Bacteroides, Lactobacillus, Bifidobacterium, Faecalibacterium, Prevotella, Shigella, Acinetobacter species, and other bacteria in their gut microbiomes.
Not every bacterium in the gut microbiome is able to produce either TMAO or PAGln; this is why only certain groups of bacteria have been associated with these circulating metabolites in cardiovascular disease. Other shifts in bacterial microbiome populations have been observed in other chronic inflammatory diseases associated with CVD risk, such as type II diabetes, so the assessment and correction of gastrointestinal microbiome imbalance may be important not only for the prevention of CVD, but the risk of other chronic inflammatory disorders as well.
The documented associations between gastrointestinal bacteria, chronic inflammation, and metabolic diseases such as type II diabetes and cardiovascular disease highlight the need for regular assessment of not only blood parameters but also the status of the gastrointestinal system and gut microbiome. The path to the human heart may truly lie in the gut – is this true for your patient? Use the GI profiles offered by US BioTek to find out.
Bui TVA, Hwangbo H, Lai Y, Hong SB, Choi YJ, Park HJ, Ban K. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ J. 2023 Aug;53(8):499-518.
Bunker JJ, Drees C, Watson AR, Plunkett CH, Nagler CR, Schneewind O, Eren AM, Bendelac A. B cell superantigens in the human intestinal microbiota. Sci Transl Med. 2019 Aug 28;11(507):eaau9356.
Fang C, Zuo K, Fu Y, Li J, Wang H, Xu L, Yang X. Dysbiosis of Gut Microbiota and Metabolite Phenylacetylglutamine in Coronary Artery Disease Patients With Stent Stenosis. Front Cardiovasc Med. 2022 Mar 25;9:832092.
Gatarek P, Kaluzna-Czaplinska J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021 Feb 11;20:301-319.
Henein MY, Vancheri S, Longo G, Vancheri F. The Role of Inflammation in Cardiovascular Disease. Int J Mol Sci. 2022 Oct 26;23(21):12906.
Liu Y, Dai M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediators Inflamm. 2020 Feb 17;2020:4634172.
Masenga SK, Hamooya B, Hangoma J, Hayumbu V, Ertuglu LA, Ishimwe J, Rahman S, Saleem M, Laffer CL, Elijovich F, Kirabo A. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J Hum Hypertens. 2022 Nov;36(11):952-959.
Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010 Aug;47(13):2170-5.
Varricchi G, Loffredo S, Borriello F, Pecoraro A, Rivellese F, Genovese A, Spadaro G, Marone G. Superantigenic Activation of Human Cardiac Mast Cells. Int J Mol Sci. 2019 Apr 12;20(8):1828.
Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. 2002 Dec;1(4):273-88.
Zhang Z, Cai B, Sun Y, Deng H, Wang H, Qiao Z. Alteration of the gut microbiota and metabolite phenylacetylglutamine in patients with severe chronic heart failure. Front Cardiovasc Med. 2023 Jan 10;9:1076806.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...