4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
6 min read
![]() Dr. Andrea Gruszecki, ND
:
July 19, 2023 at 8:30 AM
Dr. Andrea Gruszecki, ND
:
July 19, 2023 at 8:30 AM

Both external and internal conditions can result in the development of a “leaky gut”. Guidance from stool test results can help clinicians choose appropriate interventions to restore gut homeostasis and barrier functions.
Gut homeostasis and immunity occur through a complex interplay of innate and adaptive immune responses. The gut mucosa is the largest and most dynamic immunological environment of the body. It's often the first point of pathogen exposure and many microbes use it as a doorway into the rest of the body. The gut immune system therefore needs to be ready to respond to pathogens but, at the same time, is constantly exposed to innocuous environmental antigens, food particles and beneficial/commensal microflora which need to be tolerated. The term “leaky gut” has moved into common usage to describe altered intestinal permeability. While neither the term “leaky gut” nor the mechanisms that cause the problem are fully understood at present, intestinal permeability is an active area of research.
The gut mucosa is the innermost layer of the gastrointestinal tract; the mucosa lines the gut lumen, and provides an exchange interface for nutrient absorption. Gut cells (enterocytes) cooperate with cells of the intestinal immune system; they help maintain a tolerant state toward dietary and gut microbiome antigens. Tight junctions between the mucosal enterocytes are primary factor for gut barrier integrity. Immune exclusion, the prevention of antigen entry into systemic circulation, requires both a healthy gut barrier and adequate levels of secretory IgA (sIgA). A variety of important regulatory cytokines (signal molecules) are known to be secreted by enterocytes in response to stimulation by stress hormones, ingested food, pathogens, environmental toxins, minerals or vitamins.
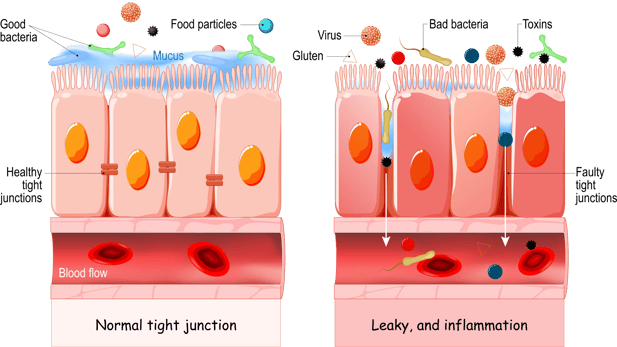
When the gut mucosa is irritated, injured, or inflamed, the mucosal enterocytes release “alarm molecules” which reduce the expression of tolerance signals and promote the release of pro-inflammatory signals that disrupt the gut barrier. Barrier disruptions increase gut “leakiness” and attract white blood cells to perpetuate local inflammation that damages the gut mucosal barrier and underlying tissues. A pro-inflammatory gut environment inflames the gut mucosa and decreases the effectiveness of the mucosal barrier. If the disruption is short-lived, the inflammation may dissipate, and homeostasis may be restored. If the disruptions continue, then the pro-inflammatory patterns may become chronic and dominate the intestinal terrain, barrier function is lost or altered, and food allergies or food intolerances may develop as the mucosal barrier loses integrity.
Fortunately, clinicians have functional stool testing to evaluate a patient’s “leaky gut” potential, as well as clinical tools to help improve intestinal permeability. Like every other health problem, the development of a leaky gut is always a combination of internal and external factors. Many of these factors will be explored in future blogs.
Stool tests, such as US BioTek’s GI Advanced Profile, can provide guidance on many of the likely causes of leaky gut, and serial testing can demonstrate improvements as therapeutic interventions restore homeostasis.
PCR microbiome mapping can detect pathogens, parasites and gut microbiome bacteria, while stool chemistries provide valuable information about gastrointestinal function and immune status:
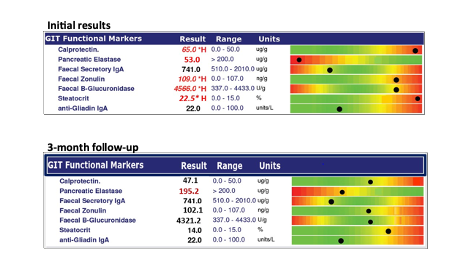
Once stool testing identifies causative factors then therapeutic interventions can be applied to restore homeostasis. A second stool test 3-4 months later can be used to ensure that the patient’s gut is responding to the interventions as intended. Based upon test results, interventions to strengthen the gut mucosal barrier and improve function may include:
Accumulating evidence indicates that gut health = human health. Guided by comprehensive tests, such as US BioTek’s GI Advanced Profile, clinicians can identify and correct many contributing causes of leaky gut.
Resources:
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014 Nov 18;14:189. https://pubmed.ncbi.nlm.nih.gov/25407511/
Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD, Keinan A, Ley RE, Gevers D, Clark AG. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015 Sep 15;16(1):191. https://pubmed.ncbi.nlm.nih.gov/26374288/
Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019 Aug;68(8):1516-1526. https://pubmed.ncbi.nlm.nih.gov/31076401/
Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020 Jun;145(6):1517-1528. https://pubmed.ncbi.nlm.nih.gov/32507229/
Chapman TP, Hadley G, Fratter C, Cullen SN, Bax BE, Bain MD, Sapsford RA, Poulton J, Travis SP. Unexplained gastrointestinal symptoms: think mitochondrial disease. Dig Liver Dis. 2014 Jan;46(1):1-8. https://pubmed.ncbi.nlm.nih.gov/23768727/
Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016 May 4;2:16003. https://pubmed.ncbi.nlm.nih.gov/28721242/
de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015 May 15;6:223. https://pubmed.ncbi.nlm.nih.gov/26029209/
De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional Keys for Intestinal Barrier Modulation. Front Immunol. 2015 Dec 7;6:612. https://pubmed.ncbi.nlm.nih.gov/26697008/
Faure M, Mettraux C, Moennoz D, et al. Specific Amino Acids Increase Mucin Synthesis and Microbiota in Dextran Sulfate Sodium–Treated Rats. J. Nutr. 2006;136:1558–1564. https://pubmed.ncbi.nlm.nih.gov/16702321/
Fukui H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm Intest Dis. 2016 Oct;1(3):135-145. https://pubmed.ncbi.nlm.nih.gov/29922669/
Hidalgo-Liberona N, González-Domínguez R, Vegas E, Riso P, Del Bo' C, et al. Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability. J Agric Food Chem. 2020 Nov 4;68(44):12476-12484. https://pubmed.ncbi.nlm.nih.gov/33084335/
Hoshiko H, Zeinstra GG, Lenaerts K, Oosterink E, Ariens RMC, Mes JJ, de Wit NJW. An Observational Study to Evaluate the Association between Intestinal Permeability, Leaky Gut Related Markers, and Metabolic Health in Healthy Adults. Healthcare (Basel). 2021 Nov 19;9(11):1583. https://pubmed.ncbi.nlm.nih.gov/34828628/
Karl J, Margolis L, Madslien E, Murphy N, Castellani J, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiologic stress. Am J Physiol - Gastrointestinal and Liver Physiology. 2017;312(6):G559-G571. https://pubmed.ncbi.nlm.nih.gov/28336545/
Kunisawa J, Kiyono H. Vitamin-mediated regulation of intestinal immunity. Front Immunol. 2013 Jul 11;4:189. https://pubmed.ncbi.nlm.nih.gov/23874335/
Lam KW, Leeds J. How to manage: patient with a low faecal elastase. Frontline Gastroenterol. 2019 Nov 15;12(1):67-73.https://pubmed.ncbi.nlm.nih.gov/33489070/
Liew WP, Mohd-Redzwan S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front Cell Infect Microbiol. 2018 Feb 26;8:60. https://pubmed.ncbi.nlm.nih.gov/29535978/
McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. 2017 Jul;278(1):277-295. https://pubmed.ncbi.nlm.nih.gov/28658542/
Ou J, DeLany J, Zhang M, Sharma S, O’Keefe S. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutrition and Cancer. 2012;64(1):34-40. https://pubmed.ncbi.nlm.nih.gov/22136517/
Pogozhykh D, Posokhov Y, Myasoedov V, Gubina-Vakulyck G, Chumachenko T, et al. Experimental Evaluation of Food-Grade Semi-Refined Carrageenan Toxicity. Int J Mol Sci. 2021 Oct 16;22(20):11178. https://pubmed.ncbi.nlm.nih.gov/34681837/
Szymanska E, Wierzbicka A, Dadalski M, Kierkus J. Fecal Zonulin as a Noninvasive Biomarker of Intestinal Permeability in Pediatric Patients with Inflammatory Bowel Diseases-Correlation with Disease Activity and Fecal Calprotectin. J Clin Med. 2021 Aug 30;10(17):3905. https://pubmed.ncbi.nlm.nih.gov/34501351/
Twardowska A, Makaro A, Binienda A, Fichna J, Salaga M. Preventing Bacterial Translocation in Patients with Leaky Gut Syndrome: Nutrition and Pharmacological Treatment Options. Int J Mol Sci. 2022 Mar 16;23(6):3204. https://pubmed.ncbi.nlm.nih.gov/35328624/
Wilson ID. (1990) Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition, Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. https://pubmed.ncbi.nlm.nih.gov/21250045/
Yel L. Selective IgA Deficiency. J Clin Immunol. 2010;30(1):10-16. https://www.ncbi.nlm.nih.gov/books/NBK538205/
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016 Apr 29;352(6285):565-9. https://pubmed.ncbi.nlm.nih.gov/27126040/

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...