4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
May 18, 2022 at 8:00 AM
Dr. Andrea Gruszecki, ND
:
May 18, 2022 at 8:00 AM

As COVID-19 social distancing practices are relaxed, new COVID-19 infections continue to be reported. There have been several disturbing reports regarding long-term post-infection consequences, including new evidence that COVID-19 infections may contribute to long-term changes in neurophysiology, glucose-insulin dysregulation, and increased autoimmune risks. The evidence for neurological changes is available in our recent CME webinar “LONG COVID-19: Unravelling the neurological consequences”. Clinicians must be alert to the possibility of post-COVID autoimmunity, because it does not follow the typical gender distribution for most autoimmune disorders – after a COVID-19 infection, the risk of autoimmune disease is highest in men.
It's no secret that the majority of autoimmune conditions affect women, with females making up 78% of autoimmunity patients. While several autoimmune conditions specifically target men, such as myocarditis, Wegener granulomatosis, idiopathic pulmonary fibrosis, diabetes, and ankylosing spondylitis, most autoimmune conditions target women. Surprisingly, men may be at a higher risk of post-COVID autoimmunity than women. There is currently no consensus regarding the cause of the gender skewing in autoimmune disorders, however gender-based differences in the expression of certain types of immune response receptors vary in men and women. However, at least one of these receptors, toll-like receptor 9 (TLR9), participates in innate viral responses, has been implicated in several autoimmune conditions and is highly expressed in women.
A quick review of the primary COVID-19 risk factors gives the first clue about male predominance in COVID autoimmunity; COVID-19 infection risk factors include male gender, increasing age, and the presence of pre-existing chronic inflammatory conditions such as obesity, diabetes, heart disease, etc. The increased risk is primarily due to infection-associated tissue damage. COVID-19 infection inflames the vascular system, reducing capillary circulation of oxygen and nutrients. If prolonged, the loss of circulation in small vessels and capillaries results in tissue damage and cell death. Hypoxia and cell damage both result in the release of pro-inflammatory mediators that can result in both local and systemic inflammation.
While early studies indicated that only severe COVID-19 cases had an increased risk of autoimmunity, later studies suggest that even men with mild COVID-19 infection may carry increased autoimmunity risk. So, what is going on?
The answer may lie in the liver. COVID-19 infection affects multiple organs, including the liver. Much of this liver damage occurs in the liver sinusoidal cells that normally induce tolerance and homeostasis, much like the gut mucosal surface. The sinusoid damage affects normal cross-talk on the liver-gut-microbiome axis. The damage has metabolic effects because the liver controls glucose/insulin metabolism, fat metabolism, and much of the body’s amino acid interconversion and metabolism. Multiple lines of evidence support liver involvement in post-COVID-19 autoimmunity, and the atypical male gender skewing:
Damage to liver tissues may suppress other hepatic regulatory functions. Suppression of these liver regulatory functions may further predispose COVID-19 survivors for autoimmunity. The liver is the primary organ that removes circulating Ig (antibody) immune complexes. Higher circulating levels of auto- and environmental antibodies are common in autoimmune conditions; their deposition into joints and tissues results in pain, local inflammation and joint stiffness. The antibody complexes bind to, and are removed from, circulation by the liver receptor FcγRIIb2. While normally inhibitory and tolerant, FcγRIIb2 dysfunction develops with the occurrence of fatty liver fibrosis (damage), inflammation or other comorbid autoimmune conditions.
Both liver and immune system functions are reliant upon healthy mitochondrial function. Mitochondrial dysfunction occurs when there are increased levels of oxidative stress, inflammatory signaling, hypoxia and tissue damage Once on a dysfunctional path, it is difficult to re-establish normal mitochondrial function without external intervention. US BioTek’s dried urine Organic Acids profile can provide an overview of mitochondrial function, NAD+ and antioxidant status. Once interpreted, a supportive nutritional protocol can be developed:
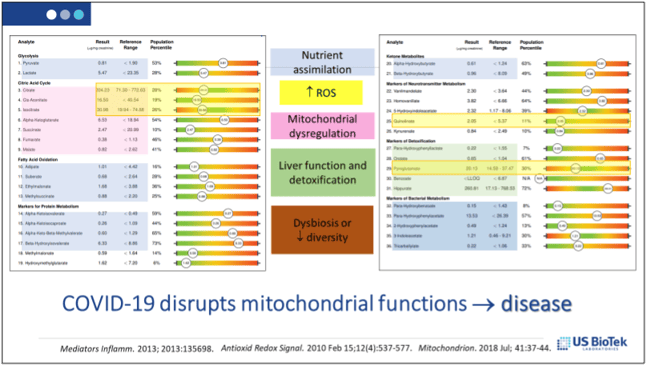
Decreasing the overall levels of histamine and circulating antibodies may also help prevent or minimize symptoms of autoimmunity. Avoidance of allergy (IgE/IgG4) or sensitivity (IgG/A) antigens, may decrease histamine and circulating antibody-antigen complexes from environmental sources:
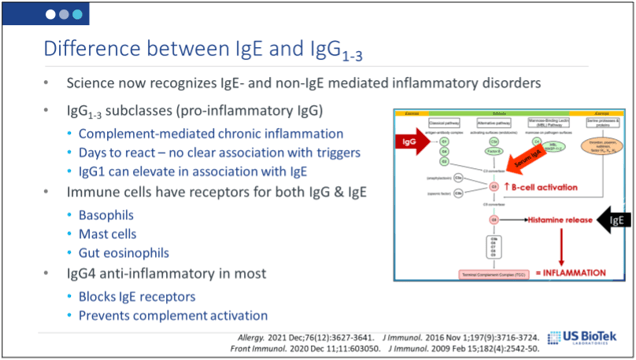
Environmental (food or inhalant) antibody levels can be evaluated via IgE/IgG4 allergy or IgG/A sensitivity testing.
After a COVID-19 infection, mild or severe, there is an increased risk of developing neurological after-effects, metab olic syndrome/type II diabetes or an autoimmune disease. The autoimmune risks appear to be highest in men, and that risk may be increased by pre-exisiting inflammatory disorders, new onset metabolic syndrome or disruption of the gut-liver axis.
Clinicians must be aware of these risks and use all the tools at their disposal to evaluate such patients, such as US BioTek’s allergy and sensitivity testing and the Organic Acids profile. Decreasing environmental antibody excess, and supporting mitochondrial function, can decrease inflammation and improve health, immune tolerance and clinical success in the treatment of COVID-19 survivors.
References
De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74-85.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...