4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
September 27, 2023 at 8:00 AM
Dr. Andrea Gruszecki, ND
:
September 27, 2023 at 8:00 AM

Chronic fatigue syndrome (CFS) is a vaguely defined disorder characterized by persistent unexplained relapsing physical/mental fatigue worsened by exertion. It is differentiated from other fatigue syndromes due to the exertional exacerbation of symptoms. In addition to the classis CFS symptoms, many patients also complain of gastrointestinal symptoms such as altered bowel habits, abdominal pain or bloating, etc. In patients with these complaints stool testing, in addition to thorough physical exam and patient history, is a necessary part of patient assessment.
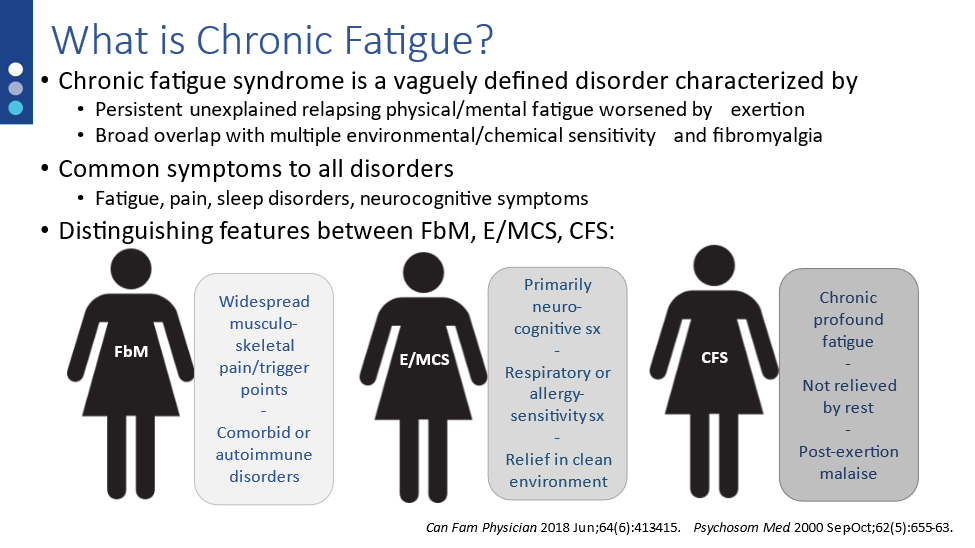
Clinicians may not realize how closely CFS is associated with gastrointestinal disorders. Different studies estimate that 35-90% of CFS patients have symptoms consistent with irritable bowel syndrome (IBS). In fact, many CFS patients report a previous diagnosis of IBS prior to the development of their CFS disorders. There are multiple reasons why this may be so, including loss of gut microbiome diversity (dysbiosis), pathogen exposure, or loss of gut barrier function.
Loss of gut microbiome diversity (dysbiosis)
A healthy microbiome contains approximately 1,000-1,500 different types of bacteria along with a variety of different types of fungus, viruses, parasites, and bacteriophages. While identifying and classifying individual bacteria from a stool sample is important and certain bacteria have been associated with disease states, from a functional perspective the presence and activity of larger groups (guilds) of bacteria is far more important for host health. A bacterial guild may contain a variety of different groups of bacteria, all of whom serve the host and the larger gut ecosystem by performing a common function. Common functions may include protein metabolism, fiber breakdown, biochemical transformation, short-chain fatty acid (SCFA) production, and immune system “cross-talk” molecules that influence health, tolerance, and inflammatory responses. Just like individual bacteria, the guilds can be adversely affected by stress, poor diet, pathogen infection or toxic exposures.
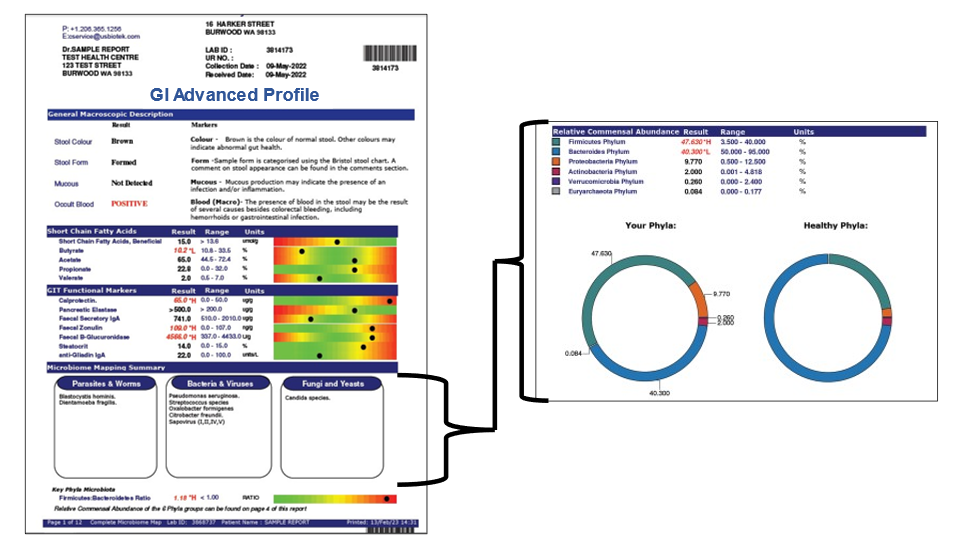
Dysbiosis occurs when the gut microbiome is disrupted and specific bacteria or guilds beneficial to the host are replaced by opportunistic bacteria and dysfunctional bacterial metabolism. Typically, a dysbiotic gut microbiome has higher levels of potentially pathogenic enterotoxin- and endotoxin-producing Proteobacteria than Firmicutes phylum bacteria, and fewer overall beneficial species. The opportunistic bacteria rarely support the host with cross-talk molecules, vitamin synthesis or assimilation. Some dysbiotic bacteria may provoke immune responses that can result in gut mucosa inflammation. Inflammation of the gut lining can decrease nutrient assimilation, disrupt immunotolerance and normal gut barrier functions and result in a “leaky gut”. Dysbiosis has been associated with chronic fatigue and many other human ailments including diabetes, cardiovascular disease, obesity, inflammatory bowel disease, and certain cancers.
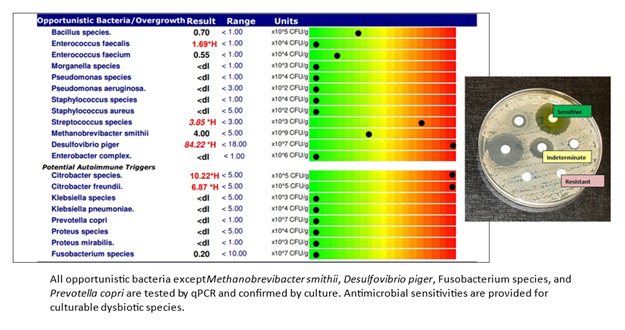
A loss of diversity in one of the primary phyla of bacteria shifts the Bacteroides/Firmicutes ratio, which can serve as a “red flag” for deeper issues. Fecal metagenomic studies identify bacteria and their metabolic pathways in study subjects. These studies have identified distinct changes in the gut microbiomes of CFS patients, CFS patients with IBS, and normal controls. Specifically, changes included decreased numbers of Firmicutes bacteria, including Faecalibacterium prausnitzii.
Pathogen exposure
Exposure to pathogenic bacteria results in gastroenteritis (diarrhea, abdominal cramps, vomiting, etc.). Most immunocompetent adults can restore normal gut homeostasis and microbiome diversity after a transient infection. In certain individuals, however, such infections can trigger a chronic dysregulation in the immune system. Prior gastrointestinal infection is a risk factor for both IBS and CFS. Pathogens known to increase these risk of post-infection IBS include Campylobacter jejuni, Salmonella enterica, Shigella sonnei, Escherichia coli 0157:H7, and norovirus. Some, such as Giardia lamblia have been associated with an increased risk for both CFS and IBS.
Loss of gut barrier function (Leaky gut)
Both the gastrointestinal microbiome and the nutritional status of the host further modulate gut homeostasis. The beneficial/commensal bacteria, mucin layer and epithelial mucosal cell barriers are not just physical and chemical barriers to pathogenic infection but represent a clear communication system resulting in direct modulation of host-driven immune responses.
If the wrong bacteria populate the microbiome, or the right bacteria are lost, the “cross-talk” signals between the gut lining and the bacteria change and the gut mucosa can become irritated and inflamed. An irritated mucosal lining will develop gaps between cells that can allow larger molecules or whole microbes into systemic circulation. Large molecules, such as undigested food molecules, can act as antigens and stimulate inflammatory responses from the immune system.
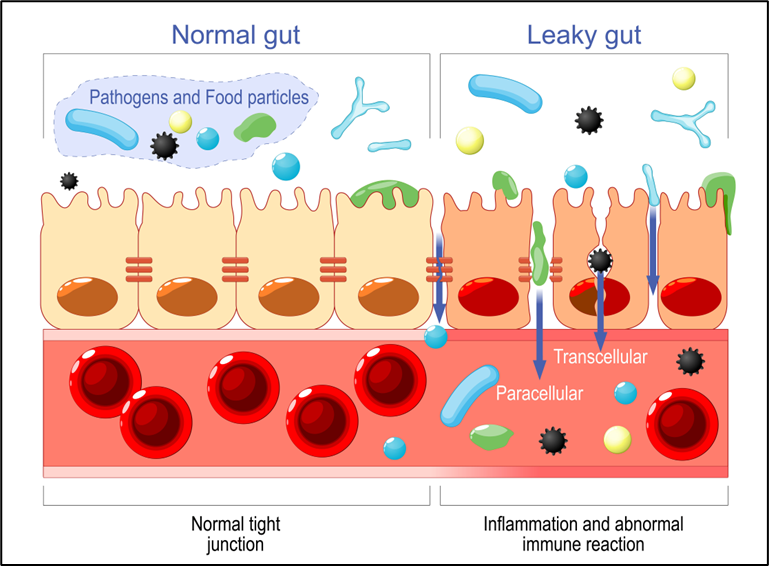
The gut mucosa can also be inflamed by pathogen exposure, poor diet, toxic chemicals (food additives, colorants, etc.), mold mycotoxins, or toxic metals. Once the gut mucosal barrier is “leaky” inflammatory compounds, such as lipopolysaccharide (LPS or “endotoxin”) from dysbiotic or pathogenic gram-negative bacteria can escape into circulation. The host immune system must function properly to remove the endotoxins from body tissues and the circulation. If they are not removed the endotoxins will continue to cause inflammation in the body. Once past the blood-brain barrier, endotoxin is particularly inflammatory and difficult to remove. Endotoxin in the brain has been associated with chronic neuroinflammation.
Evidence supports the presence of “leaky guts” in CFS patients. Studies demonstrate the presence of circulating antibodies against the endotoxins from Pseudomonas aeruginosa, Morganella morganii, Proteus mirabilis, Pseduomonas putida, Citrobacter koseri, and Klebsiella pneumoniae. Another study found that, in CFS patients, exercise challenge shifted the populations of bacteria in the gut microbiome and increased circulating levels of inflammatory endotoxins from Clostridia and Bacilli species which persisted for days (compared to normal controls). Both the change in bacterial populations and the excess endotoxin are thought to contribute to the post-exertion fatigue experienced by CFS patients.
Conclusion
The presence of leaky gut, persistent pathogens, dysbiotic bacteria, gastrointestinal inflammation, along with other biomarkers of gastrointestinal function and the status of normal bacterial gut flora, can be assessed with US BioTek’s new Advanced GI Profile. Consider screening all chronic fatigue patients, all patients with chronic GI symptoms, and all patients with chronic inflammatory disorders to discover and correct gut problems that may cause or exacerbate chronic fatigue syndrome and other inflammatory conditions.
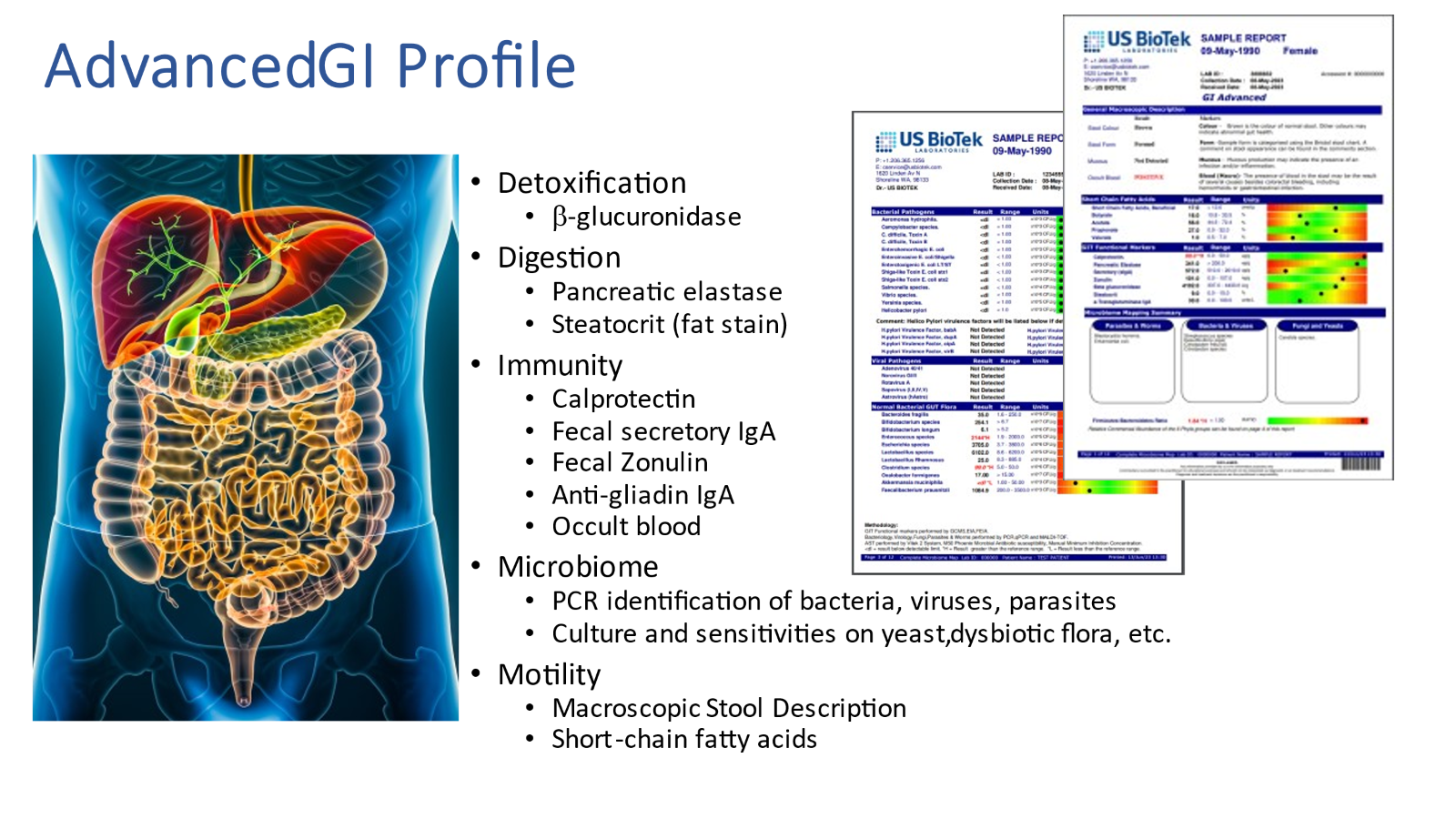
References:
Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. 2019 Sep 13;16(1):180. doi: 10.1186/s12974-019-1564-7.
https://pubmed.ncbi.nlm.nih.gov/31519175/
Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020 Jun;145(6):1517-1528.
https://pubmed.ncbi.nlm.nih.gov/32507229/
de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015 May 15;6:223.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4432792/
Gao Y, Meng L, Liu H, Wang J, Zheng N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins (Basel). 2020 Sep 28;12(10):619.
https://pubmed.ncbi.nlm.nih.gov/32998222/
Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond). 2010 Oct 12;7:79.
https://pubmed.ncbi.nlm.nih.gov/20939923/
Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007 Oct;98 Suppl 1:S29-35.
https://pubmed.ncbi.nlm.nih.gov/17922955/
Nagy-Szakal D, Williams BL, Mishra N, Che X, Lee B, Bateman L, Klimas NG, Komaroff AL, Levine S, Montoya JG, Peterson DL, Ramanan D, Jain K, Eddy ML, Hornig M, Lipkin WI. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017 Apr 26;5(1):44. doi: 10.1186/s40168-017-0261-y.
https://pubmed.ncbi.nlm.nih.gov/28441964/
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019 Oct 7;11(10):2393. https://pubmed.ncbi.nlm.nih.gov/31591348/
Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, Frank DN. Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS One. 2015 Dec 18;10(12):e0145453.
https://pubmed.ncbi.nlm.nih.gov/26683192/
Varesi A, Deumer US, Ananth S, Ricevuti G. The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. J Clin Med. 2021 Oct 29;10(21):5077.
https://pubmed.ncbi.nlm.nih.gov/34768601/

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...