4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
4 min read
![]() Dr. Andrea Gruszecki, ND
:
March 9, 2021 at 8:00 AM
Dr. Andrea Gruszecki, ND
:
March 9, 2021 at 8:00 AM

The development of successful COVID-19 vaccines is the beginning of the end of the COVID-19 pandemic. As more and more individuals in the general population get vaccinated, “herd immunity” is developed, and herd immunity can help protect even those who choose not to receive the vaccine. New testing, using antibodies against the spike protein receptor binding domain (S-RBD) protein used in vaccines, is available from US BioTek. This new antibody test can tell the difference between a COVID-19 infection and protection from a successful COVID-19 vaccination.
S-RBD stands for "spike protein receptor binding domain." The COVID-19 S-RBD binds to the ACE2 receptor and allows the virus access to the body's cells. Higher levels of S-RBD antibodies have been shown to block or "neutralize" the COVID-19 virus from getting into cells and causing infection.
Individuals who have recovered from a COVID-19 viral infection usually have high levels of antibodies specific to S-RBD. The "neutralizing" ability of S-RBD antibodies is why all three of the Emergency Use Authorized (EUA) vaccines target the S-RBD, although they use different methods for getting the S-RBD protein information into the body.
While human studies indicate that spike protein and nucleocapsid protein antibodies elevate in response to COVID-19 virus exposure, these antibodies are not as specific as the S-RBD antibodies for preventing COVID-19 infection (or re-infection) or for confirming post-vaccination status. After the virus infects the human body, it creates a large quantity of both “neutralizing” antibodies such as S-RBD and many nonspecific endogenous antibodies.
While a general trend of post-infection increases in spike and nucleocapsid antibodies in association with increased S-RBD antibodies has been observed, measurement of the generalized antibodies is not always predictive of S-RBD status, particularly when the only stimulus is an S-RBD vaccine:
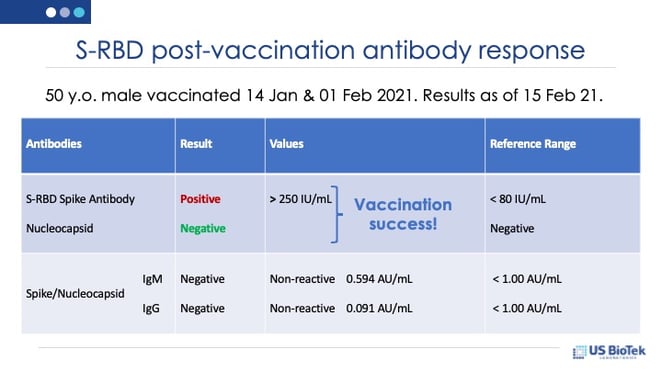
There are several good reasons to measure antibody status before and after a COVID-19 vaccination. Pre/post-vaccination assessment is the only way to be sure that a COVID-19 vaccination was effective. First, not everyone can mount an adequate antibody response to either COVID-19 infection or vaccination, and it is important to protect those that cannot make the needed antibodies.
Second, not everyone needs to be vaccinated immediately. Individuals with natural antibodies from a COVID-19 infection do not require immediate vaccination. After infection, a vaccination only becomes necessary when natural antibody levels begin to drop 3-6 months later. Serial testing can help determine when it’s the right time to vaccinate.
The COVID-19 Immune Response Panel measures both nucleocapsid (N-protein) and S-RBD antibodies. N-protein antibodies do not rise in response to COVID-19 S-RBD vaccinations. The combined serum IgM/IgG tests measure the body’s response to COVID-19 nucleocapsid (N) and spike protein receptor binding domain (S-RBD) exposure. The N and S-RBD antibodies' combination assay has a > 99% positive and negative predictive value 15 days after COVID-19 exposure. This test does not differentiate between IgM and IgG; studies indicate that joint testing is as or more reliable than individual IgM and IgG testing in confirming COVID-19 exposure.
| Interpretation | N-Antibody IgM/IgG | S-RBD Antibody IgM/IgG | |
| Negative | Non-reactive | Non-Reactive | Consider COVID-19 RT-PCR if indicated |
| Equivocal | Reactive | Non-Reactive | Possible COVID-19 exposure. Consider COVID-19 RT-PCR if indicated. |
| Inoculation-Responsive | Non-Reactive | Reactive | Successful response to inoculation by either vaccine or COVID-19 virus. Consider COVID-19 RT-PCR if indicated. |
| Positive | Reactive | Reactive | Exposure to COVID-19 Virus within the last 6 months. Consider COVID-19 RT-PCR if indicated. |
The COVID-19 Vaccine Response Screen measures only the antibody response to the S-RBD protein. Once the baseline immune response has been established, this test can be used post-vaccination to evaluate immune status. The average time for seroconversion has been estimated at 2-3 weeks. However, the strongest antibody responses will likely be seen after the second vaccine dose 6-8 weeks after the first inoculation.
In conclusion
Not everybody needs to be vaccinated right away because those with natural COVID-19 antibodies are protected until antibody levels decline. Those who need to be vaccinated right away can be evaluated before vaccination to screen for a current or prior COVID-19 infection and determine a baseline antibody status. After vaccination, individuals can be evaluated to measure their post-vaccination response, ensuring they have reached adequate protection from future COVID-19 exposures. Don’t guess - assess patient status pre and post vaccination to be sure!
References:
Centers for Disease Control and Prevention
Germain N, Herwegh S, Hatzfeld AS, Bocket L, Prévost B, Danzé PM, Marchetti P. Retrospective study of COVID-19 seroprevalence among tissue donors at the onset of the outbreak before implementation of strict lockdown measures in France. Cell Tissue Bank. 2021 Feb 1. doi: 10.1007/s10561-021-09901-3.
Griffin S. (30 Dec 2020) COVID-19: Antibodies protect against reinfection for at least six months, study finds. BMJ 2020;371:m4961
Hall V, et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv 2021.01.13.21249642; doi: https://doi.org/10.1101/2021.01.13.21249642 Accessed 12 Feb 2021.
Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis. 2011 Sep;43(9):683-9.
Ledford H. (14 January 2021) COVID reinfections are unusual — but could still help the virus to spread. Nature. https://www.nature.com/articles/d41586-021-00071-6#correction-0 Accessed 12 Feb 2021.
Moore JP, Offit PA. (2021) SARS-CoV-2 Vaccines and the Growing Threat of Viral Variants. JAMA. doi:10.1001/jama.2021.1114 Accessed 01 Mar 21 https://jamanetwork.com/journals/jama/fullarticle/2776039
Premkumar L, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020 Jun 11;5(48):eabc8413.
Sampath Kumar NS, Chintagunta AD, Jeevan Kumar SP, Roy S, Kumar M. Immunotherapeutics for Covid-19 and post vaccination surveillance. 3 Biotech. 2020 Dec;10(12):527. doi: 10.1007/s13205-020-02522-9.
Sewell H. (17 Dec 2020) Covid-19 vaccines: delivering protective immunity. BMJ 2020; 371 doi: https://doi.org/10.1136/bmj.m4838 Accessed 12 Feb 2021.
Zhou Z, Wang X, Fu Y, Zhang X, Liu C. Letter to the editor: Neutralizing antibodies for the treatment of COVID-19. Acta Pharm Sin B. 2021 Jan;11(1):304-307.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...