4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
April 21, 2020 at 3:16 PM
Dr. Andrea Gruszecki, ND
:
April 21, 2020 at 3:16 PM

In the effort to get all those who need COVID-19 testing done, many laboratories, including US BioTek, are stepping up to provide in-office testing for clinicians and their patients. There are two different types of testing available: RT-PCR and serum IgM/IgG.
Each test provides a different kind of information:
RT-PCR stands for Real-Time Reverse Transcriptase Polymerase Chain Reaction. This is a term for taking a strand of viral RNA (Coronavirus is an RNA virus) and turning it into a strand of RNA/DNA hybrid. The use of hybrids increases the speed of the replication process needed to get a readable result. Three separate genetic markers are evaluated by this test; if two of the three markers are present, the result is positive and there is COVID-19 virus in the patient’s cells. RT-PCR is the Centers for Disease Control (CDC) -recommended diagnostic test for COVID-19 for the current Coronavirus pandemic. The test methodology is well-established and similar to RT-PCR tests used for seasonal influenza.
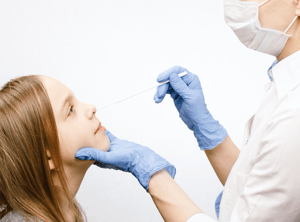
The accuracy of the RT-PCR test depends, in part, on the type of specimen submitted for evaluation. The CDC recommends a nasopharyngeal swab specimen collected by a healthcare worker, although other acceptable specimens include mid-turbinate swabs, anterior nasal swabs, and oropharyngeal swabs collected by healthcare workers.
Order Now: COVID-19 Diagnostic Testing
A second form of testing is serum IgM/IgG. This type of sample requires a blood draw and centrifuge of the specimen prior to shipping. The serum IgM/IgG does not directly test for the virus, but instead measures the body’s immune response to Coronavirus exposure. Predictions and interpretation of serum IgM/IgG are based in human studies of antibody responses during the severe acute respiratory syndrome-related Coronavirus (SARS) epidemic and current studies of patients in China.
After infection, the immune system develops antibodies to fight the virus exposure. The presence of IgM/IgG antibodies is a strong indication that COVID-19 exposure has occurred. The IgM antibodies begin to rise three to five days after virus exposure but may not be high enough for detection until seven to 10 days have passed. The IgG antibodies begin to rise three to four weeks after a Coronavirus exposure. It is possible to be exposed to COVID-19 and have no symptoms, yet still develop an antibody response. Because of individual variability in immune responses, and the lag time in antibody responses, antibody testing is not used independently to diagnose the presence of COVID-19. The antibody test is used to confirm a positive RT-PCR result or indicate immunity.
IgM antibodies can rise even if there is a negative or inconclusive RT-PCR test; if this happens an RT-PCR retest is still indicated to confirm the diagnosis. Poor specimen collection may sometimes cause a false-negative RT-PCR result. The presence of both IgM and IgG antibodies may indicate a recent mild or symptom-free Coronavirus exposure or the recovery phase of a Coronavirus illness. IgG antibodies may remain elevated for several years, while IgM antibodies will likely drop below the cutoff value within a month. Of note, IgG antibodies may also indicate prior exposure to other Coronavirus strains (HKU1, NL63, OC43, or 229E), as well as immunity to COVID-19 or other Coronaviruses.
Because the IgM and IgG antibodies rise and fall at different times, the relationship between the two different antibody levels provides information about when an exposure took place and where the patient is in the recovery process. Ideally, the serum antibodies are tested along with an RT-PCR to provide the maximum amount of diagnostic information:
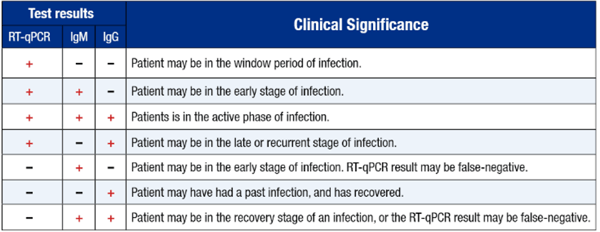
Of note, antibody response may be poor in immunocompromised individuals or patients on immunosuppressive drugs, and may be lower than expected in some pediatric patients. Alternately, if it is very early in the infection process, the virus may be present, but the immune system is still upregulating antibody production. For these reasons, a negative response (no antibodies) does not rule out a Coronavirus infection, nor does it protect against future infection. If a patient has symptoms consistent with COVID-19 infection or if they have worsening symptoms, consider a repeat RT-PCR for confirmation using a nasopharyngeal swab specimen or retest antibodies in a few more days.
Order Now: COVID-19 Antibody Testing
Currently, the recommended diagnostic test for COVID-19 is the RT-PCR. However, if swab collection is difficult, or if there is a history of COVID-19-like symptoms and subsequent recovery, or if there are concerns regarding a patient’s ability to mount an effective immune response against the Coronavirus, consider either a combination test for both RT-PCR and serum IgM/IgG or just antibody testing to prevent delays in receiving useful clinical results.
Consider RT-PCR testing or antibody testing for:
The last category of patients that may require RT-PCR testing consists of those patients currently in quarantine. While there are CDC guidelines for releasing patients from quarantine based on their symptom remission, such guidelines may not reflect the most recent data from Wuhan, China, which indicates that the COVID-19 virus sheds for an average of 20 days after the onset of symptoms. The longest record of viral shedding so far is 37 days, however, had this patient survived they would have shed virus for a longer time. With this information, it is easy to see that the test-based quarantine release strategy recommended by the CDC is best able to keep the general population safe from infection.
Under the test-based strategy symptomatic patients may be released from quarantine when:
The increasing availability of COVID-19 testing provides clinicians with the opportunity to make informed decisions regarding the care and isolation of patients with COVID-19 symptoms, and patients recovering from infection. Increased COVID-19 screening may help decrease transmission rates and may improve care for infected patients.
Diazyme Laboratories, Inc. http://www.diazyme.com/dz-lite-sars-cov-2 Accessed 06 April 2020.
Kai-Wang To K, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet. 23 March 2020. DOI:https://doi.org/10.1016/S1473-3099(20)30196-1.
ThermoFisher Scientific https://www.thermofisher.com/us/en/home/clinical/clinical-genomics/pathogen-detection-solutions/coronavirus-2019-ncov/genetic-analysis/taqpath-rt-pcr-covid-19-kit.html Accessed 06 April 2020.
Woo PC et al. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Emerg Infect Dis. 2007;13(10):1562–1564.
Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Lancet. 2004 Mar 13; 363(9412):841-5.
www.CDC.gov Discontinuation of Isolation for Persons with COVID-19 Not in Healthcare Settings (Interim Guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html Accessed 06 April 2020.
www.CDC.gov Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html Accessed 06 April 2020.
www.CDC.gov People Who Are at Higher Risk for Severe Illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fspecific-groups%2Fpeople-at-higher-risk.html Accessed 06 April 2020.
Zhao LQ, et al. Serological analysis of SARS Coronavirus in children diagnosed clinically as severe acute respiratory syndrome cases during SARS epidemic in Beijing. Zhonghua Er Ke Za Zhi. 2006 Apr; 44(4):262-6.
Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 11 March 2020;395(10229):1054-1062.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...