50 Inhalant Panel
Our 50 Inhalant IgE Panel expertly assesses antibody responses to 50 airborne allergens, including trees, grasses, mold, and dust, aiding in the management of a range of allergy symptoms from minor to severe. Utilizing superior chemiluminescence technology for enhanced sensitivity and accuracy, our panel ensures effective allergy management.
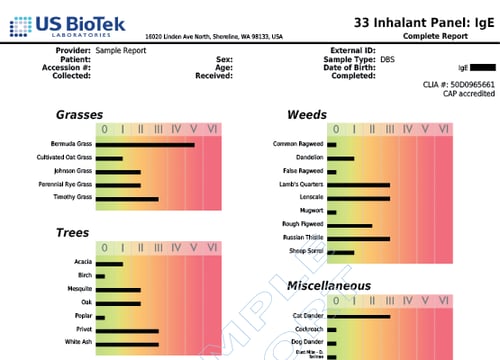
- GRASSES
- MISCELLANEOUS
- MOLDS
- TREES
- WEEDS

This test is run on Serum samples.
| IgE | |
| Serum Requirements | 4 ml |

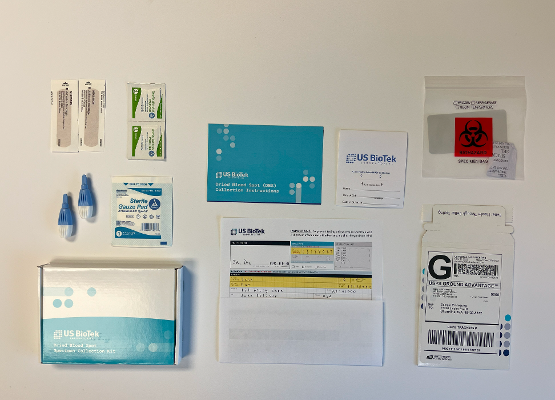
4 Simple Steps to Results

Steps 1
ORDER THE KIT
Collection Kits are free for US BioTek account holders and ship within 1 business day
Steps 2
COLLECT SAMPLE
Kits are designed for the simplest & most hygienic collection process possible

Steps 3
SHIP SAMPLE
Prepaid return shipping labels are included making shipping simple

Steps 4
RECIEVE RESULTS
Results are delivered to the provider portal with industry leading turnaround times.
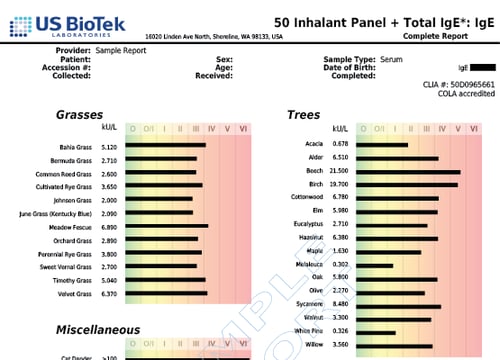
Grasses
- Bahia Grass
- Bermuda Grass
- Common Reed Grass
- Cultivated Rye Grass
- Johnson Grass
- June Grass (Kentucky Blue)
- Meadow Fescue
- Orchard Grass
- Perennial Rye Grass
- Sweet Vernal Grass
- Timothy Grass
- Velvet Grass
Miscellaneous
- Cat Dander
- Cockroach
- Cow Dander
- Dog Dander
- Horse Dander
- Dust Mite - D. farinae
- Dust Mite - D. pteronyssinus
- House Dust
Molds
- Aspergillus fumigatus
- Alternaria fenuis/Alternata
- Candida albicans
- Cladosporium herbarum
- Penicillium notatum
Trees
- Acacia
- Alder
- Beech
- Birch
- Cottonwood
- Elm
- Eucalyptus
- Hazelnut
- Maple
- Melaleuca
- Oak
- Olive
- Sycamore
- Walnut
- White Pine
- Willow
Weeds
- Common Ragweed
- Dandelion
- English Plantain
- Goldenrod
- Lamb’s Quarters
- Mugwort
- Nettle
- Rough Marsh Elder
- Russian Thistle
Markers Tested
Grasses
- Bahia Grass
- Bermuda Grass
- Common Reed Grass
- Cultivated Rye Grass
- Johnson Grass
- June Grass (Kentucky Blue)
- Meadow Fescue
- Orchard Grass
- Perennial Rye Grass
- Sweet Vernal Grass
- Timothy Grass
- Velvet Grass
Miscellaneous
- Cat Dander
- Cockroach
- Cow Dander
- Dog Dander
- Horse Dander
- Dust Mite - D. farinae
- Dust Mite - D. pteronyssinus
- House Dust
Molds
- Aspergillus fumigatus
- Alternaria fenuis/Alternata
- Candida albicans
- Cladosporium herbarum
- Penicillium notatum
Trees
- Acacia
- Alder
- Beech
- Birch
- Cottonwood
- Elm
- Eucalyptus
- Hazelnut
- Maple
- Melaleuca
- Oak
- Olive
- Sycamore
- Walnut
- White Pine
- Willow
Weeds
- Common Ragweed
- Dandelion
- English Plantain
- Goldenrod
- Lamb’s Quarters
- Mugwort
- Nettle
- Rough Marsh Elder
- Russian Thistle
VeriTek Process

AUTOMATED SYSTEMS

SUPERIOR ASSAY PREPARATION

DUPLICATE TESTING

EXTERNAL ACCOUNTABILITY
Need Assistance With
Specimen Collection?
Accurate results start with proper specimen collections. Watch the video or download detailed instructions to walk you through the collection process.